Arrhenius postulated the concept of acid. According to him "The substance that produces hydrogen ions in aqueous solution is known as acid." Interpretation: The definition of each term, Arrhenius acid, Bronsted-Lowry acid and Lewis acid is to be given. The most general definition from these...Describes the Arrhenius, Bronsted-Lowry, and Lewis theories of acids and bases, and explains the relationships between them. Hydrochloric acid is neutralised by both sodium hydroxide solution and ammonia solution. In both cases, you get a colourless solution which you can crystallise to get a white...A) An Arrhenius acid is a substance that increases the concentration of hydronium ion in water. B) An Arrhenius acid reacts with a base to produce a salt and water.An Arrhenius acid is a substance whose aqueous solutions give rise to hydrogen ions or protons. And thus we may identify as an acid a species that undergoes the following reaction with water... And so an Arrhenius acid must contain an hydrogen ion that can be transferred to solvent....An Arrhenius acid is a compound that increases the H+ ion concentration in aqueous solution. Some Arrhenius Acids FORMULA NAME HC2H3O2 (also written CH3COOH) Acetic acid HClO3 Chloric acid HCl What can be inferred from the given statement? a. Ca(OH)2 accepts H+ ions...
Theories of acids and bases
Arrhenius defined acids as substances that increase the concentration of hydrogen ions when added to water. One of the oldest definitions used in the study of acid base chemistry is one derived by Svante August Arrhenius in the late 1800s.We define Arrhenius acidity with respect to water, but what will water itself be? It is both an Arrhenius acid and an Arrhenius base and thus the only Arrhenius amphoteric compound. Asking for help, clarification, or responding to other answers. Making statements based on opinion; back...An Arrhenius acid is a substance that contains hydrogen and produces hydrogen ions in solution. The dissociation constant of a weak acid HA and weak base BOH are 2 × 10−5 and 5 × 10−6 respectively. The equilibrium constant for the neutralization reaction of the two is:(ignore hydrolysis of...Terms in this set (59) acids to produce salts and water. Which statement about Arrhenius acids is FALSE? They increase the concentration of hydroxide ions in aqueous solution.

[Solved] Which of the following statements does not... | Course Hero
Definition of Arrhenius acids and bases, and Arrhenius acid-base reactions. Arrhenius acids and bases. This is the currently selected item.Which statement describes the Arrhenius interpretation of acids and bases? It is limited to situations that involve aqueous solutions or specific Which characteristic best identifies an Arrhenius base? It must generate hydroxide ions in water. Why is citric acid added to food? To add tartness.Recall that an Arrhenius acid is defined as a proton, H+1, donor in water, and an Arrhenius base Because the complete definitions of Arrhenius acids and bases were not utilized to develop the "HX Based on the criteria that are described in the previous paragraph, both the types of ions that are...An Arrhenius acid is a substance that, when dissolved in water, increases the concentration of hydronium ion, H3O+(aq). The Arrhenius concept limits bases to compounds that contain a hydroxide ion. The Brønsted-Lowry concept expands the compounds that can be considered acids...2. Which statement best describes a characteristic of acid solutions? (a) They react with some metals to form hydrogen gas. The student will: • define an Arrhenius acid and list some substances that qualify as acids under this definition.
Yahoo Answers is shutting down on May 4th, 2021 (Eastern Time) and starting April 20th, 2021 (Eastern Time) the Yahoo Answers site will likely be in read-only mode. There will be no changes to other Yahoo properties or services and products, or your Yahoo account. You can find extra information about the Yahoo Answers shutdown and methods to obtain your knowledge in this lend a hand web page.
Acid and Base Equilibrium - [PPT Powerpoint]
![Acid and Base Equilibrium - [PPT Powerpoint] Acid and Base Equilibrium - [PPT Powerpoint]](https://i0.wp.com/reader012.vdocuments.site/reader012/slide/20180224/5681473c550346895db47b82/document-12.png?t=1592574492)
Quiz6.docx - Question 1 1 1 pts of all the amino acids how ...

Which statement describes the citric acid cycle - Which ...

PPT - Acids & Bases CHM 1046 Bushra Javed Valencia College ...
PPT - Answers in red. PowerPoint Presentation - ID:3197197

Which salts will be more soluble in an aci... | Clutch Prep

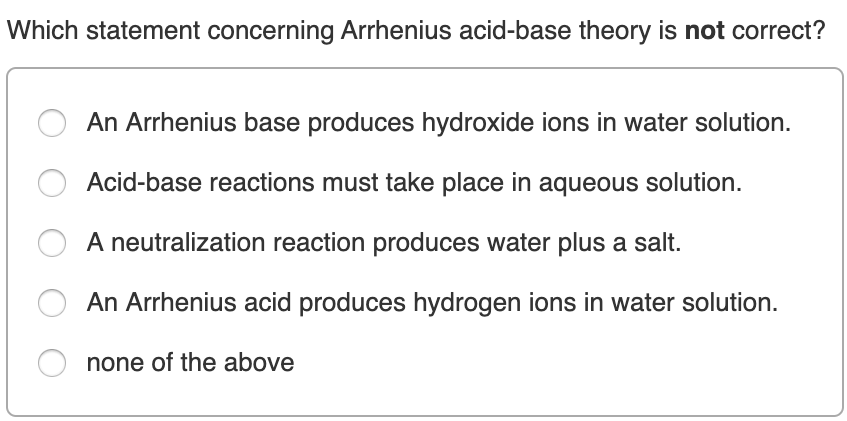
Which statement concerning Arrhenius acid-base theory is ...
According to the table of results which of the following ...

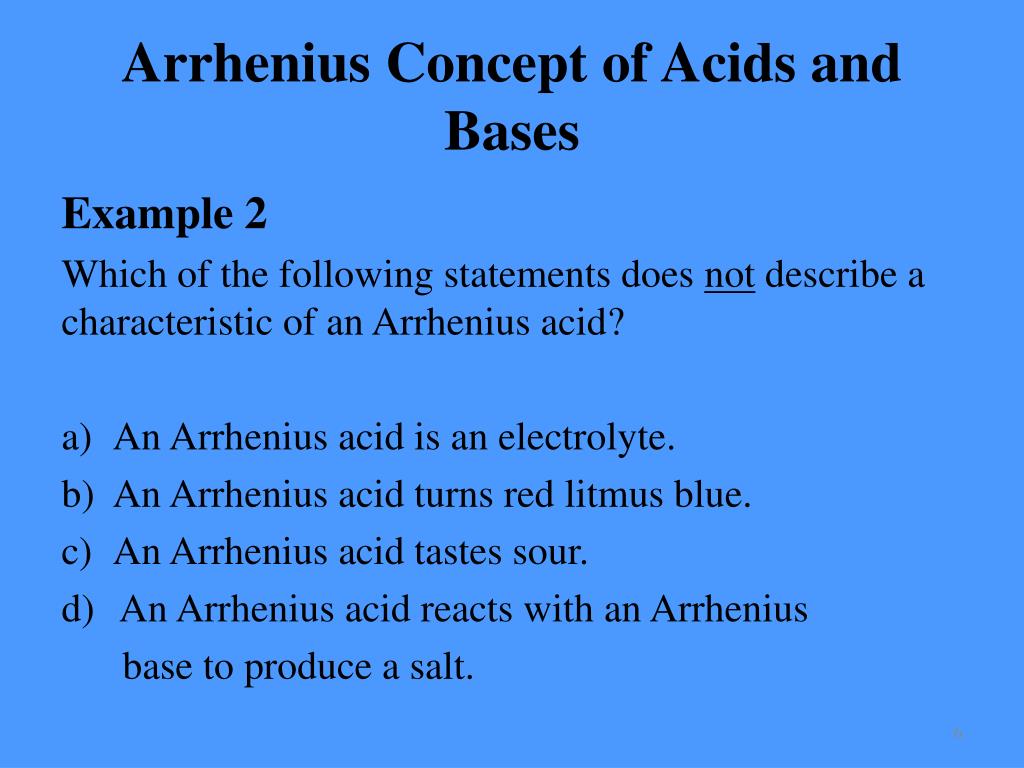
Solved: Which Of The Following Statements Does Not Describ ...
Which statement describes the citric acid cycle - Which ...

Which statement best describes ABCs acid test ratio for ...

Chemistry Archive | May 09, 2016 | Chegg.com

PPT - June 2010 Regents Exam Part A, B1 and B2 Questions ...

Solved: *Which Statement BEST Describes The Role Of Water ...

whitten10e_tb_ch10.doc - Chapter 10Reactions in Aqueous ...

Acid and Base Equilibrium - [PPT Powerpoint]
![Acid and Base Equilibrium - [PPT Powerpoint] Acid and Base Equilibrium - [PPT Powerpoint]](https://i0.wp.com/reader012.vdocuments.site/reader012/slide/20180224/5681473c550346895db47b82/document-37.png?t=1592574492)
Solved: 1) Which Statement Best Describes How An Enzyme Lo ...

Solved: 1) Which Statement Concerning Arrhenius Acid-base ...

Solved: Which Of The Following Statements Best Describes T ...

Which of the following describes a disaccharide It has ...

Acid and Base Equilibrium - [PPT Powerpoint]
![Acid and Base Equilibrium - [PPT Powerpoint] Acid and Base Equilibrium - [PPT Powerpoint]](https://i0.wp.com/reader012.vdocuments.site/reader012/slide/20180224/5681473c550346895db47b82/document-18.png?t=1592574492)
0 comments:
Post a Comment